How Many Electrons Can the F Orbital Hold
Thus the fourth level can hold up to 32 electrons. The fourth and higher levels have an f sublevel in addition to the s p and d orbitals.
How Many F Orbitals Are There What Are They Quora
The f subshell has a total of seven orbitals and each orbital can hold two electrons and so the f subshell can hold a total of 72 14 electrons.
. The third principal energy level has one s orbital three p orbitals and five d orbitals which can each hold up to 10 electrons. The suborbitals are denoted by a numeral or number before the orbital thus. Each orbital can hold only two electrons.
Note that the first shell can hold 2 electrons and the second shell up to 8. How many electrons are in each orbital shell. The first orbit only has an S orbital.
The first shell has one 1s orbital and holds 2 electrons. What is the maximum number of d orbital in a. Each orbital can hold no more than two electrons.
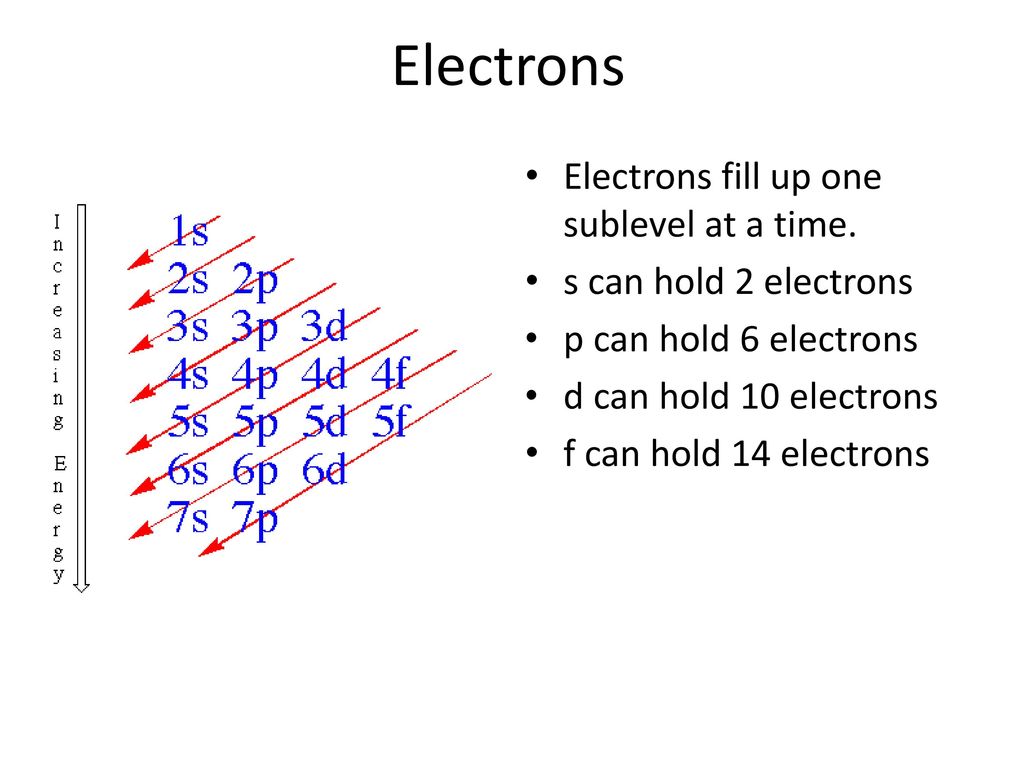
The first shell has one 1s orbital and holds 2 electrons. To calculate electron shell capability you first need to determine the number of electrons possible per shell then apply the 2n 2 formula. This way that the s orbital can contain increase to 2 electrons the ns orbital can contain up to six electrons the d orbital deserve to contain as much as 10 electrons and the f orbital deserve to contain up to 14 electrons.
The f sublevel as a whole can hold up to 14 electrons due to the fact that it consists of 7 orbitals but each one can only hold up to 2 electrons. 1 s 2 s 2 p 4 s 3 d 4 p These are just examples of the way. 1 orbital 2 electrons.
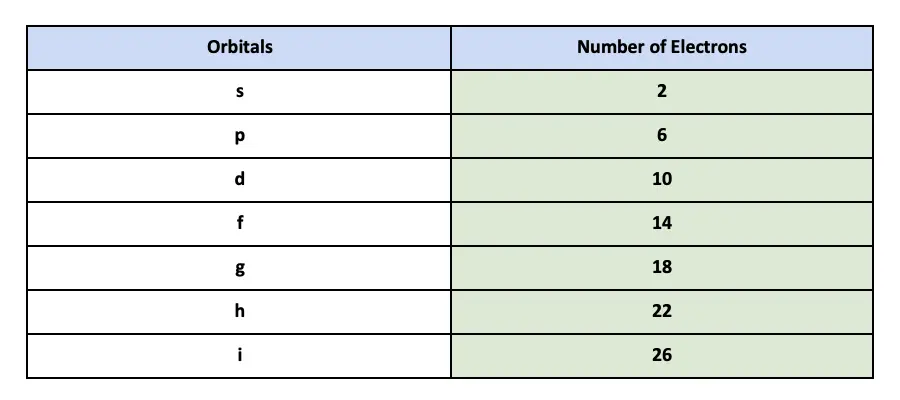
The 2p3p 4p and 5p can each hold six electrons because they have three orbitals. 2 in the s orbital 6 in the three p orbitals 10 in the five d orbitals and 14 in the seven f orbitals. How many electrons does each orbital hold.
How many electrons can 6s hold. As we know that f subshell contain 7 orbital and each orbital can hold maximum 2 electons so correct answer would be 2we can simply understand this by taking the real life example imagine that there is house named f which consists of 7 rooms so similarly in this case house is a subshell and each room is an orbital. Like all f subshells it is made up of f orbitals.
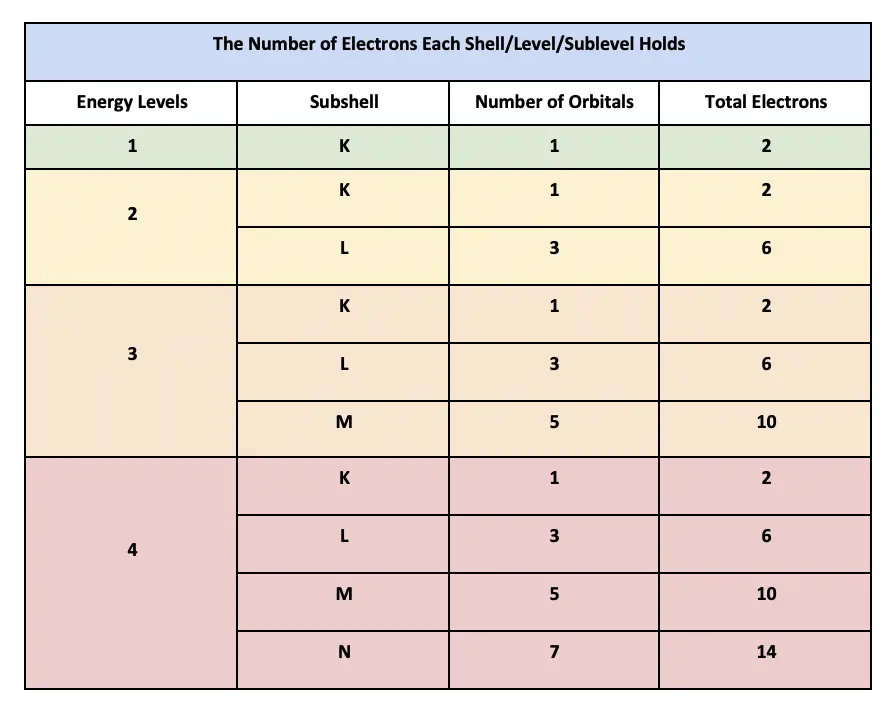
1 s-orbital and 3 p-orbitals. 7 orbitals 14 electrons. The first shell can hold up to two electrons the second shell can hold up to eight 2 6 electrons the third shell can hold up to 18 2 6 10 and so on.
This means that the first shell can hold 2 electrons. How many electrons can f orbitals hold. After that the fifth sixth and seventh energy levels also have four sublevels each.
The f sublevel as a whole can hold up to 14 electrons due to the fact that it consists of 7 orbitals but each one can only hold up to 2 electrons. Every subshell has a of orbits spdf that can each hold 2 electrons each one has the opposite spin of the other. The fifth energy level contains.
Any orbital holds only 2. One spin-up and one spin-down. Also the d sublevel has 5 orbitals and thus can hold a maximum of 10 electrons in it and that of the sub level has 7 orbitals which can hold 14 electrons in its shell.
S subshellp subshelld subshellf subshell Table 1. 5s which holds 2 electrons 5p which holds 6 5d holds 10 and 5f holds 14 for a total of 32 electrons. Each f subshell holds at most 14 electrons.
Click to see full answer Herein how many electrons can 4d hold. Each orbital holds two electrons which differ in a property known as spin. How many electrons can f orbitals hold.
Similarly the p sublevel has 3 orbitals and can hold a total of 6 electrons in its shell. How many orbitals are in the sub level d. However the electron can exist in spin up m s 12 or with spin down m s -12 configurations.
Is the third shell 8 or 18. The number of electrons in a 4f subshell can be anything between 0 if it isnt filled and 14 2 electrons per orbital times 7 orbitals 14 electrons. The second shell holds.
The subshells s p d and f contain the following number of orbitals respectively where every orbital can hold up to two electrons maximum. Failure and nature of Subshells Visualizing Electron Orbitals. The first shell of all atoms has 1 subshell of s-orbitals containing 1 s orbital.
So each s sublevel can have two electrons each p sublevel can hold six electrons etc. 5 orbitals 10 electrons. Orbital has 2 electrons max an f orbital can hold 14 electrons.
How many orbitals are in the sub level f. Each orbital can hold up to two electrons meaning that the 1s 2s 3s 4s and 5s can hold two electrons. How many electrons can fit into one orbital.
What is the maximum number of orbitals. This means that the 1s 2s 3s 4s etc can each hold two electrons because they each have only one orbital. How many can each orbital hold.
How many electrons are in an orbital. Each orbital can hold two electrons. How many electrons can each orbital shell hold.
An electron will always try to enter the orbital with the lowest energy. The s subshell has 1 orbital that can hold up to 2 electrons the p subshell has 3 orbitals that can hold up to 6 electrons the d subshell has 5 orbitals that hold up to 10 electrons and the f subshell has 7 orbitals with 14 electrons. 3 orbitals 6 electrons.
The f orbital will always be 14 electrons at maximum. How many electrons can a 4f orbital hold. Any orbital can hold a maximum of 2 electrons with opposite spin.
The sublevels of the first four principal energy levels and the maximum number of electrons that the sublevels can contain are summarized in. Why does the third shell have 8 electrons. The second shell has 2 subshells.
Any orbital can hold a maximum of 2 electrons with opposite spin. Electrons that occur together in an orbital are called an electron pair. Each orbital can hold 2 electrons one of each spin and a maximum of 7 f orbitals can have the same energy without violating Paulis exclusion principle.
A region of space within an atom where an electron in a given subshell can be found. A 4f subshell is the lowest-energy f subshell starting at Cerium. This allows for a maximum of 18 electrons.
Two electrons Each shell can contain only a fixed number of electrons.

How Many Electrons Can The Fourth Energy Level Hold At Level
How Many Electrons Are In Each Shell Including 3p Orbitals
How Many Electrons Can The Fourth Energy Level Hold At Level

Quantum Theory And The Electronic Structure Of Atoms Chapter Ppt Download
Parsing The Spdf Electron Orbital Model

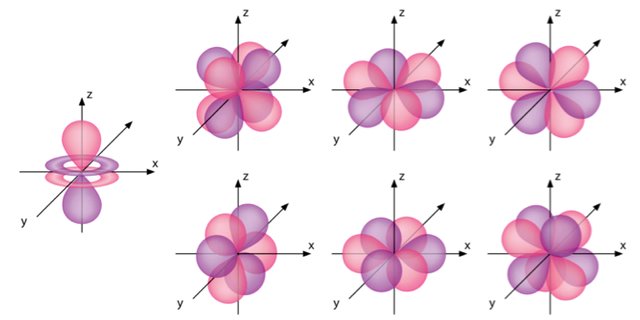
What Is The Shape Of An F Orbital Quora

P Subshell Can Accommodate A Maximum Of How Many Electrons
Parsing The Spdf Electron Orbital Model

Fluorine Orbital Diagram Electron Configuration And Valence Electron

Shapes Of S P D And F Orbitals Chemistry Problems Facebook
Electrons Orbitals Ppt Download
How Many Electrons Are In Each Shell Including 3p Orbitals

The Actinide Research Quarterly 1st Quarter 2004
How Many Electrons Are In Each Orbital Spdf Quora
How Do Atomic Shells And Subshells Explain The Periodic Table Quora

Electron Orbitals Shapes Subshells Names Video Lesson Transcript Study Com

Comments
Post a Comment